- Product Details
Keywords
- Flibanserin HCL
- Flibanserin Hydrochloride
- BIMT-17
Quick Details
- ProName: Flibanserin HCL// Flibanserin Hydrochl...
- CasNo: 147359-76-0
- Molecular Formula: C20H21F3N4O.HCL
- Appearance: white powder
- Application: treatment for pre-menopausal women wit...
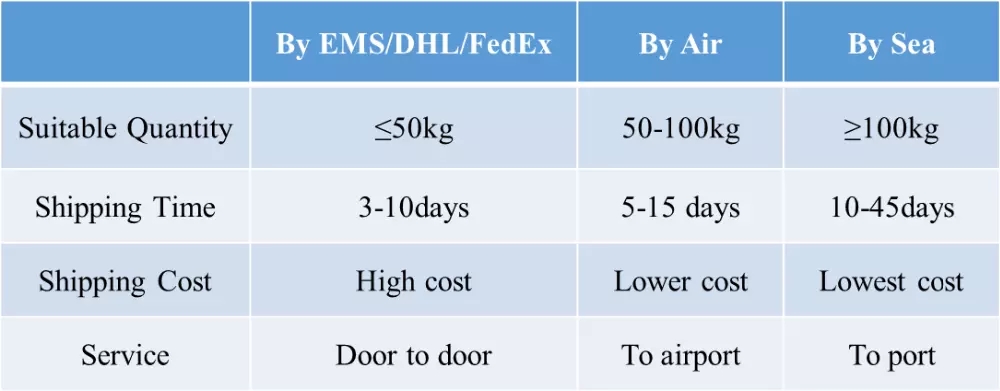
- DeliveryTime: immediately after payment receives
- PackAge: discreet packing
- Port: HK, Shanghai, Shenzhen
- ProductionCapacity: 500 Kilogram/Month
- Purity: 99%
- Storage: cool and dry place
- Transportation: EMS,DHL,HK post,EUB,ETK,NL post,German...
- LimitNum: 10 Gram
Superiority
1.high quality:
quality is life. quality is the most important element for all goods. we have a lab doing research in wuhan china. hplc and nmr is available if needed.
2.reasonable price:
we provide high quality products with competitive price in china. all customers are welcomed to send us inquiries and get quotation.
3.low moq:
no worry about the low moq, our moq is 1 gram or even lower.
4.good service.
fast response. we promise to reply within 24 hours including holidays and send quotation sheet and other documents within 48 hours.
5. fast shipping and secure courier.
we promise to send out products and provide tracking number within 3 working days. and we send via different couriers based on different destination countries. we usually use nl post, hk post, germany post, eub, etk, etc.

Details
Flibanserin HCL // Flibanserin Hydrochloride /BIMT-17 99.0%min powder
Product Name: Flibanserin Hydrochloride
Chemical Formula: C20H22ClF3N4O
Molecular Weight: 426.86
CAS:147359-76-0
Character: white powder
Purity (HPLC): 99.0%
Flibanserin Test standard:
| Test Items | Test Specifications | Test Results |
| Appearance | An off-white to white powder | Complied |
| Identification | Meet the requirements | Positive |
| Loss on Drying | ≤0.50% | 0.13% |
| Heavy Metals | ≤20PPM | <20PPM |
| Purity (by HPLC) | ≥99.0% | 99.2% |
| Conclusion: | Conform to standard |
Flibanserin HCL (INN, USAN) (developmental code name BIMT-17; proposed trade names Girosa and Addyi) is a drug that is being studied as a non-hormonal treatment for pre-menopausal women with hypoactive sexual desire disorder (HSDD). Development by Boehringer Ingelheim was halted in October 2010 following a negative evaluation by the U.S. Food and Drug Administration. The rights to the drug were then transferred to Sprout Pharmaceuticals, which is continuing the drug development process. On June 4, 2015, the panel to the FDA recommended approval of the drug by 18–6.

Packing and shipping

 Premiumsupplier
Premiumsupplier


