- Product Details
Keywords
- terbinafine HCl
- terbinafine hydrochloride
- cas 78628-80-5
Quick Details
- ProName: terbinafine hydrochloride / terbinafin...
- CasNo: 78628-80-5
- Molecular Formula: C20H26Cl2N2O
- Appearance: white powder
- Application: improved memory and increased learning...
- DeliveryTime: immediately
- PackAge: aluminum foil bag
- Port: HK, Shanghai, Shenzhen
- ProductionCapacity: 100 Kilogram/Month
- Purity: 99%
- Storage: cool and dry place
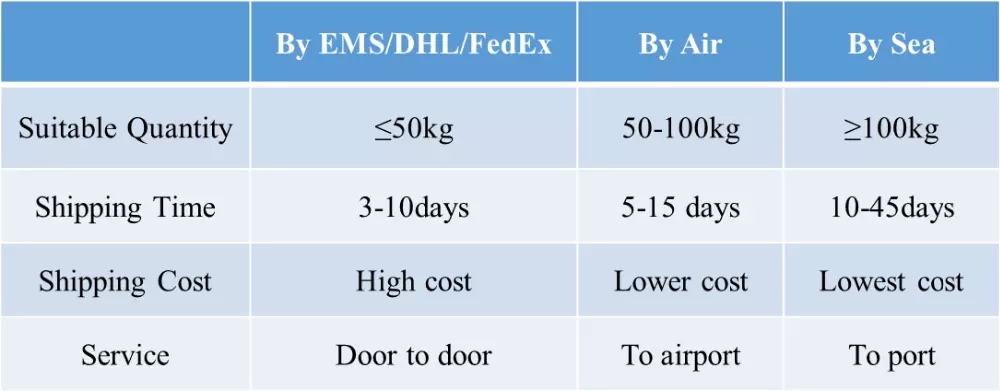
- Transportation: EMS,EUB,FEDE, DHL, UPS,etc
- LimitNum: 10 Gram
Superiority
1.high quality:
quality is life. quality is the most important element for all goods. we have a lab doing research in wuhan china. hplc and nmr is available if needed.
2.reasonable price:
we provide high quality products with competitive price in china. all customers are welcomed to send us inquiries and get quotation.
3.low moq:
no worry about the low moq, our moq is 1 gram or even lower.
4.good service.
fast response. we promise to reply within 24 hours including holidays and send quotation sheet and other documents within 48 hours.
5. fast shipping and secure courier.
we promise to send out products and provide tracking number within 3 working days. and we send via different couriers based on different destination countries. we usually use nl post, hk post, germany post, eub, etk, etc.
Details
terbinafine hydrochloride / terbinafine HCl CAS 78628-80-5
Function
It was initially researched as a treatment for allergies and inflammatory disorders, particularly asthma, but despite being well tolerated in clinical trials and showing reasonable efficacy against allergen-induced airway responses in asthmatic patients, it failed to show sufficient advantages over existing drugs and was discontinued from further development in this application. However, following the discovery in 2012 that the prostaglandin D2 receptor (DP/PGD2) is expressed at high levels in the scalp of men affected by male pattern baldness, the rights to setipiprant were acquired by Kythera with a view to potentially developing this drug as a novel treatment for baldness, with a previously unexploited mechanism of action. While it is too early to tell whether setipiprant will be an effective treatment for this condition, the favorable pharmacokinetics and relative lack of side effects seen in earlier clinical trials mean that fresh clinical trials for this new application can be conducted fairly quickly. A phase 2A study is underway to evaluate the safety, tolerability, and efficacy of oral setipiprant relative to a placebo and the active comparator, finasteride, in 18 to 41 years old males with androgenetic alopecia.
Applications
It was initially researched as a treatment for allergies and inflammatory disorders, particularly asthma, but despite being well tolerated in clinical trials and showing reasonable efficacy against allergen-induced airway responses in asthmatic patients, it failed to show sufficient advantages over existing drugs and was discontinued from further development in this application. However, following the discovery in 2012 that the prostaglandin D2 receptor (DP/PGD2) is expressed at high levels in the scalp of men affected by male pattern baldness, the rights to setipiprant were acquired by Kythera with a view to potentially developing this drug as a novel treatment for baldness, with a previously unexploited mechanism of action. While it is too early to tell whether setipiprant will be an effective treatment for this condition, the favorable pharmacokinetics and relative lack of side effects seen in earlier clinical trials mean that fresh clinical trials for this new application can be conducted fairly quickly. A phase 2A study is underway to evaluate the safety, tolerability, and efficacy of oral setipiprant relative to a placebo and the active comparator, finasteride, in 18 to 41 years old males with androgenetic alopecia.

 Premiumsupplier
Premiumsupplier