- Product Details
Keywords
- Sofosbuvir
- Sofosbuvir powder
- cas 1190307-88-0
Quick Details
- ProName: High quality anti HCV API Sofosbuvir
- CasNo: 1190307-88-0
- Molecular Formula: C22H29FN3O9P
- Appearance: white powder
- Application: Sofosbuvir is used in combination ther...
- DeliveryTime: immediately
- PackAge: aluminum foil bag
- Port: HK, Shanghai, Shenzhen
- ProductionCapacity: 100 Kilogram/Month
- Purity: 99%
- Storage: cool and dry place
- Transportation: email: wonda-chem@outlook.com
- LimitNum: 10 Gram
Superiority
1.high quality:
quality is life. quality is the most important element for all goods. we have a lab doing research in wuhan china. hplc and nmr is available if needed.
2.reasonable price:
we provide high quality products with competitive price in china. all customers are welcomed to send us inquiries and get quotation.
3.low moq:
no worry about the low moq, our moq is 1 gram or even lower.
4.good service.
fast response. we promise to reply within 24 hours including holidays and send quotation sheet and other documents within 48 hours.
5. fast shipping and secure courier.
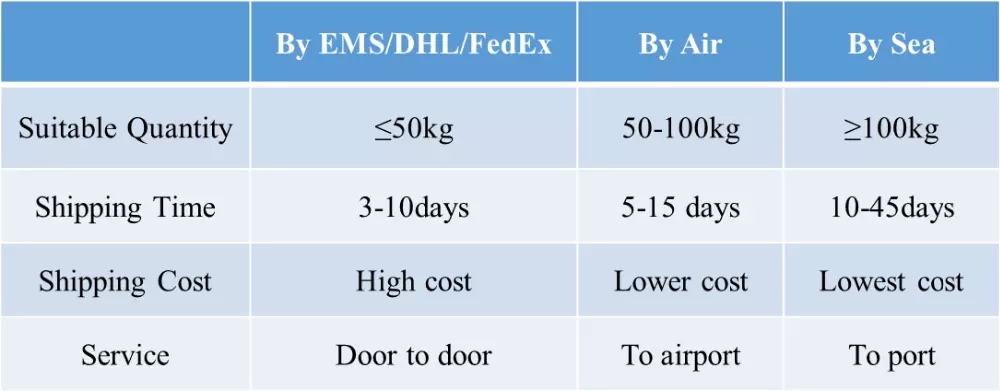
we promise to send out products and provide tracking number within 3 working days. and we send via different couriers based on different destination countries. we usually use nl post, hk post, germany post, eub, etk, etc.
Details
| Name | Sofosbuvir |
| Structure |
|
| Other name | PSI-7977, GI-7977, Sofosbuvir,propan-2-yl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate |
| CAS NO. | 1190307-88-0 |
| Molecular Formula | C22H29FN3O9P |
| Molecular Weight | 529.458 g/mol |
| Chemical and Physical Properties | |
| Appearance | White to off-white crystalline powder |
| Solubility | Slightly soluble in water ,In water, 105 mg/L at 25 deg C (est) |
| Quality standard | In-house, purity 99% |
| Stability | Stable if stored as directed; avoid strong oxidizing agents |
| Application | |
| Drug Indication | Sofosbuvir is used in combination therapy with other antiviral medications to treat chronic hepatitis C virus (HCV) infected patients with HCV genoptypes 1-6, and to treat HCV and HIV co-infected patients. |
| Formulations/Preparations | Oral: Tablets, file-coated 400 mg |
| Handling and storage | |
| Safety and Hazards | Information for safe handling: avoid inhalation and contact with skin, eyes and clothing; material may be an irritant. |
| Handling and storage | Store at room temperature below 30 deg C (86 deg F). |
| Sample package | Aluminium foil bag |
| Commercial package | 25kg Fiber drum |
| UN Code | Not classfied |
| HS Code | 29349990.90 |
| Shipping group | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| Sales restriction | NO |
| Origin | China |
| Technical Support | |
| Sofosbuvir STD Data sheet | Yes |
| Sofosbuvir MSDS | Yes |
| Sofosbuvir COA | Yes |
| Sofosbuvir HPLC | Yes |
| Sofosbuvir Transport certificate | Yes |
| Sofosbuvir DMF | Yes |
| Supply ability | |
| Monthly capacity | More than 1.5 tons |
| GMP | Factory GMP |
| Related APIs | |
| Related anti-hepatitis C virus APIS |
Daclastavir dihydrochloride Entecavir monohydrate Ledipasvir acetone Ledipasvir copovidone Velpatasvir copovidone Sofosbuvir |

 Premiumsupplier
Premiumsupplier