- Product Details
Keywords
- Ledipasvir
- Ledipasvir powder
- GS-5885
Quick Details
- ProName: Top quality 99% Ledipasvir powder GS-5...
- CasNo: 1441674-54-9
- Molecular Formula: C49H54F2N8O6.C3H6O
- Appearance: white powder
- DeliveryTime: immediately
- PackAge: aluminum foil bag
- Port: HK, Shanghai, Shenzhen
- ProductionCapacity: 100 Kilogram/Month
- Purity: 99%
- Storage: cool and dry place
- Transportation: email: wonda-chem@outlook.com
- LimitNum: 10 Gram
Superiority
1.high quality:
quality is life. quality is the most important element for all goods. we have a lab doing research in wuhan china. hplc and nmr is available if needed.
2.reasonable price:
we provide high quality products with competitive price in china. all customers are welcomed to send us inquiries and get quotation.
3.low moq:
no worry about the low moq, our moq is 1 gram or even lower.
4.good service.
fast response. we promise to reply within 24 hours including holidays and send quotation sheet and other documents within 48 hours.
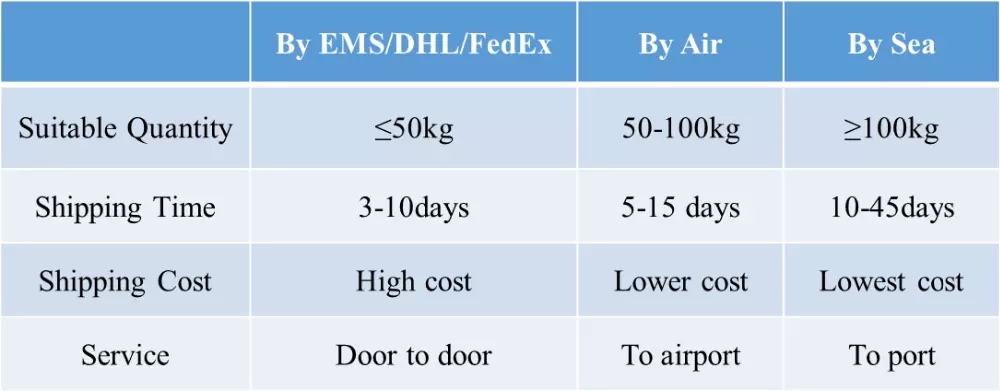
5. fast shipping and secure courier.
we promise to send out products and provide tracking number within 3 working days. and we send via different couriers based on different destination countries. we usually use nl post, hk post, germany post, eub, etk, etc.
Details
Top quality 99% Ledipasvir powder GS-5885 CAS 1441674-54-9
What is Ledipasvir
Ledipasvir is a potent inhibitor of HCV NS5A, a viral phosphoprotein that plays an important role in viral replication, assembly, and secretion, a hepatitis C virus protein.

 Premiumsupplier
Premiumsupplier