- Product Details
Keywords
- Apremilast
- Otezla
- cas 1270138-40-3
Quick Details
- ProName: Apremilast / Otezla/ Intermediates/ AP...
- CasNo: 608141-41-9
- Molecular Formula: C22H24N2O7S
- Appearance: white powder
- Application: approved for the treatment of psoriati...
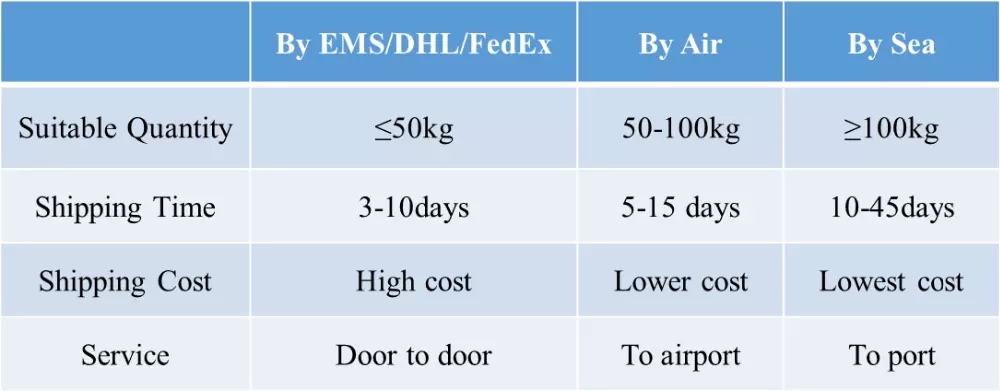
- DeliveryTime: immediately
- PackAge: aluminum foil bag
- Port: HK, Shanghai, Shenzhen
- ProductionCapacity: 100 Kilogram/Month
- Purity: 99%
- Storage: cool and dry place
- Transportation: email: wonda-chem@outlook.com
- LimitNum: 10 Gram
Superiority
1.high quality:
quality is life. quality is the most important element for all goods. we have a lab doing research in wuhan china. hplc and nmr is available if needed.
2.reasonable price:
we provide high quality products with competitive price in china. all customers are welcomed to send us inquiries and get quotation.
3.low moq:
no worry about the low moq, our moq is 1 gram or even lower.
4.good service.
fast response. we promise to reply within 24 hours including holidays and send quotation sheet and other documents within 48 hours.
5. fast shipping and secure courier.
we promise to send out products and provide tracking number within 3 working days. and we send via different couriers based on different destination countries. we usually use nl post, hk post, germany post, eub, etk, etc.
Details
Product Name: Apremilast
Form: Powder
CAS No.:608141-41-9
Molecular Fomula: C22H24N2O7S
Molecular weight: 460.51
Brand: Salus
Sample: Available
COA: Available
Apremilast was approved by the USFDA in March 2014 for treatment of adults with active psoriatic arthritis. Apremilast is the first oral agent that is FDA-approved for the treatment of psoriatic arthritis and offers the convenience of oral dosing compared to treatment with biopharmaceuticals. In September 2014, the USFDA approved Otezla (apremilast) for the treatment of moderate to severe plaque psoriasis.It is also being tested for its efficacy in treating other chronic inflammatory diseases such as ankylosing spondylitis, Behcet's disease, and rheumatoid arthritis.

 Premiumsupplier
Premiumsupplier